What is the UKCA mark?
As the UK has officially left the European Union the CE mark used to identify a product as suitable for sale within the EU will become unsuitable for use on products intended for the UK market.
The United Kingdom Conformity Assessment (UKCA) mark will be used to identify products suitable for sale within England, Scotland and Wales.
It will affect all products that fall under the current CE marking regulations, except for products that fall under the Medical Devices Directive.
When should the UKCA mark be used?
The UKCA Mark should be used immediately after 1st January 2021 where all of the following apply:
a. The product is for the market in Great Britain
b. Is Covered by legislation which requires UKCA marking
c. The product requires mandatory third-party conformance assessment
d. If the conformity assessment has been carried out by a UK conformity assessment body and the conformance files are not transferred from your UK body to an EU recognised body before 1st January 2021
For all other cases, including those who self-certify for conformity, manufacturers are not required to apply the mark until 1st January 2022. During this period of transition the CE mark will continue to be accepted in the UK.
What does the mark look like?

This mark will be applied in order to trade in England, Scotland and Wales.
You haven’t Mentioned Northern Ireland?
That is correct, as of January 2021, the Northern Ireland Protocol will be in place, and NI will align with any relevant EU rules for placing product onto the market.
This means that for most goods the CE mark will still be the accepted mark for goods placed into Northern Ireland. The UKCA mark will not be recognised within Northern Ireland but an additional mark may be required.
What is the additional mark?


So when should the UKNI mark be applied?
For any product that requires mandatory third-party conformity assessments, and it is to be performed by a UK body, then an additional marking to the CE Mark must be applied.
The UKNI mark cannot be applied alone, it must be used alongside the CE mark. However goods with the CE and UKNI mark cannot be placed on the market in the EU.
You do not need to make any changes if both of the following apply:
a. You are a manufacturer based in Northern Ireland (or are the manufacturer’s authorised representative)
b. You currently mark your goods on the basis of a supplier’s declaration of conformity, sometimes known as ‘self-certifying’
Should I remove the CE mark from my products?
This will depend on where you sell your products, if you only intend to sell product into the UK Market, then only the UKCA mark is required.
However should you be selling into both the UK and European markets, then the product will require multiple marks.
The below table highlights which mark is required for which area of the market.
| Intended Market | Conditions | Accepted Marking |
| United Kingdom | Manufactured product placed on the market until end of 2021 | UKCA or CE |
| Manufactured product placed on the market from 1st Jan 2022 | UKCA | |
| Qualifying product from Northern Ireland being placed on the UK Market with unfettered access | CE or CE and UKNI | |
| Northern Ireland | Self-Certified or third party EU Recognised Body assessment | CE |
| Third party UK based body assessment | CE and UKNI | |
| Europe | All conditions | CE |
Where should I place the new Mark?
The marking must be placed permanently on the product itself, however there is a transitional period for doing this.
It is allowable for the mark to be placed on the product or accompanying documentation until 1st January 2023. The mark can be added in the form of a label during this period.
From 1st January 2023 the mark, in most cases, must be affixed directly to the product.
The UKCA mark, must be easily visible and legible, be at least 5mm in height (unless specified by relevant legislation), and cannot be used in different proportions to the design.

What about my current product stocks?
Under the transitional period, government guidance states that if the product is fully manufactured and conformity marked before 1st January 2021, then the goods can be made available on the UK market with no changes.
Can a manufacturer apply the mark prior to 1st Jan 2021?
Yes, however it should be noted that the mark will hold no official status until 1st January 2021, so it will not be legally recognised until this time.
What other activities are needed to support the Mark?
In support of the mark a Declaration of Conformity must be created for products bearing the UKCA mark.
This document is predominantly the same as current requirements for a DoC for product entering the European mark, with a couple of key changes:
a. You must list UK regulations rather than EU
b. You must list designated British Standards rather than those listed in the European Union Official Journal
Therefore any product intended for both the UK and EU markets will require two Declaration of Conformity documents.
Will there be any Technical Difference between UK marking requirements and CE marking requirements?
The technical requirements on January 1st 2021 will for the most part be unchanged.
So, the technical file created for CE marking requirements will be sufficient to prove evidence required for the UKCA mark.
However it is important to note that regulations and requirements may change over time, as UK and EU requirements diverge.
Manufacturers should stay vigilant to ensure they are fully compliant with any changes to requirements that may arise.
It is advisable to monitor the UK Government website for up to date information.
As a manufacturer who uses third parties for conformity assessment, how can I be sure I am compliant?
As of the 1st January 2021, the following rules apply:
CE Marking based upon a certificate of conformity issued by a UK Notified Body will no longer be valid, certificates will need to be updated and re-issued by an EU notified body.
UK based EU Notified Bodies will automatically become UK Approved Bodies from 1st January 2021.
In the first instance it is advisable to contact your assessment partner and confirm any changes that may be required. Many of the UK notified bodies have been working to secure offices or representation in Europe to ensure they can update certificates of conformity.
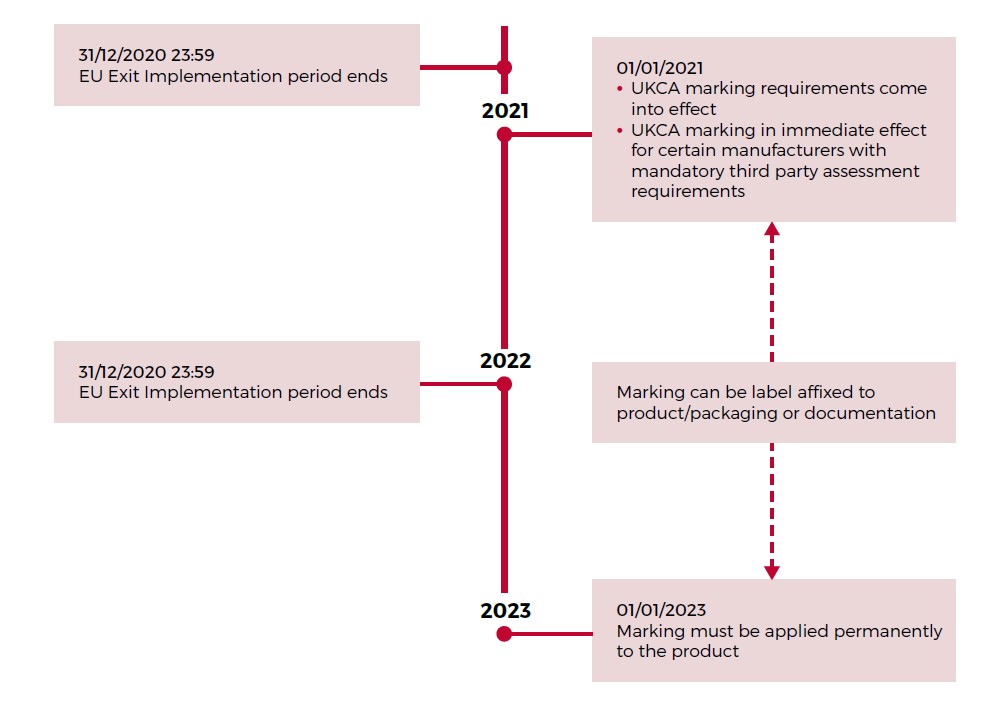
What are the Key Dates for implementation?
The UKCA implementation period, and indeed this document highlights a number of key dates.
The key milestones are shown in the below timeline.
Acknowledgements
Acknowledgment and thank you to GAMBICA for information provided to facilitate the creation of this document.
GAMBICA is the Trade Association for Instrumentation, Control, Automation and Laboratory Technology in the UK. For further information and membership details please visit the GAMIBCA website.
Seaward Electronic Ltd. have been manufacturing and supplying electrical safety test instruments to the manufacturing industry for over 35 years. To find out more about our expertise and view our full range of test equipment please click here.
Please click on the link below if you would like to download a PDF copy of this guidance.
Download as PDF
















Sign up to our Newsletter.
Stay up to date with the latest industry and product news, as well as our free educational content such as webinars and our expert guides.
Close